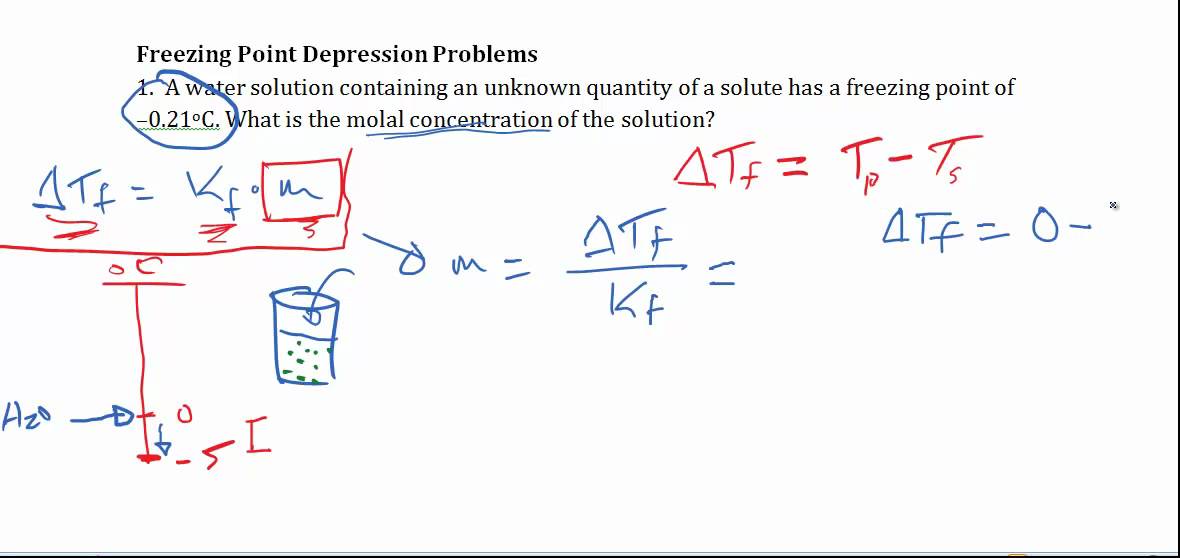
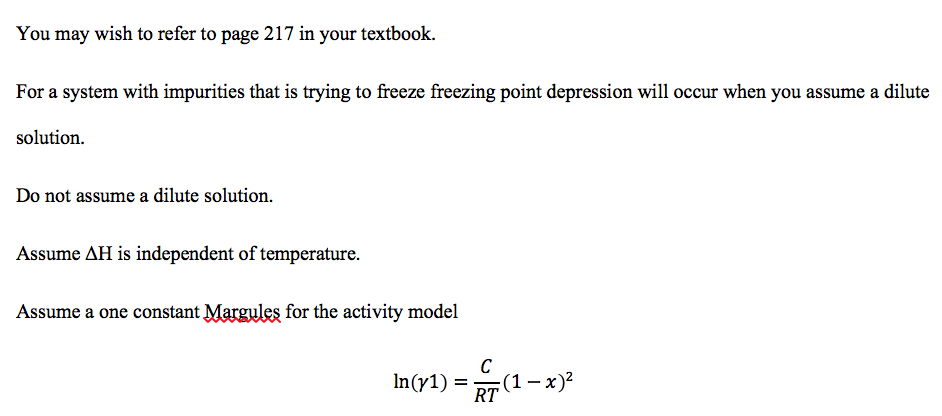
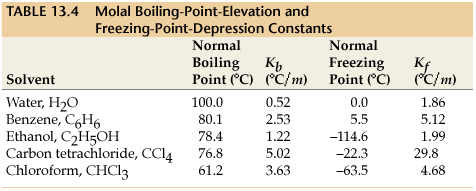
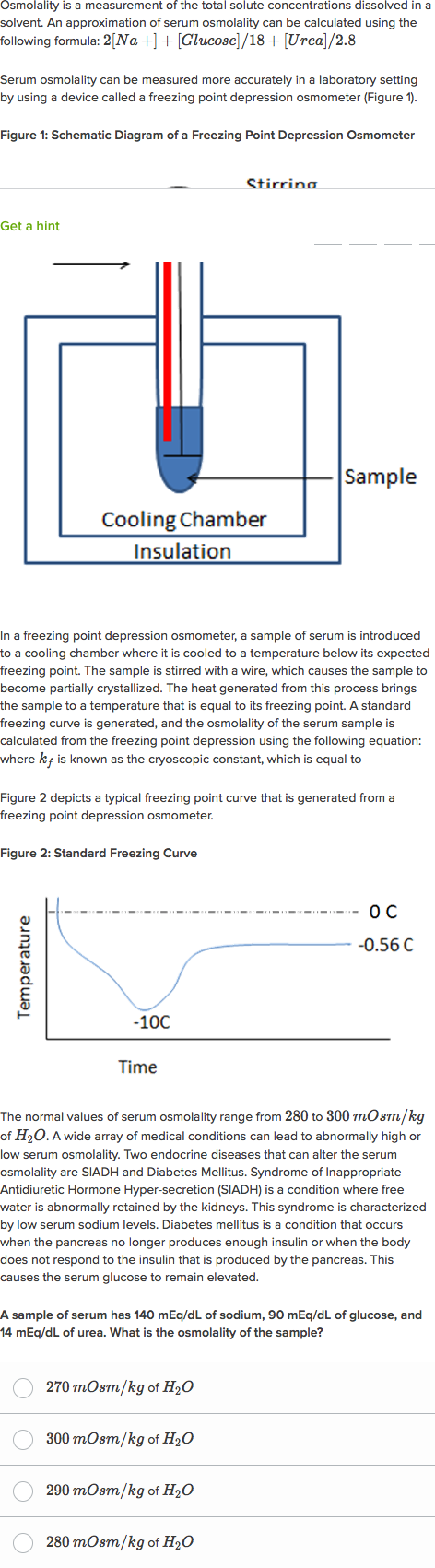
Freezing Point Depression
Get Freezing point depression For Free - Find your perfect HD wallpaper for your phone, desktop, website or more! - Free download - High-quality wallpapers - Advanced filters for searching. Today i will share Freezing point depression wallpaper. The best creative wallpapers are not cheap photos, but are works of art in their own right. Wallpapers can be creative, inspiring, gorgeous.
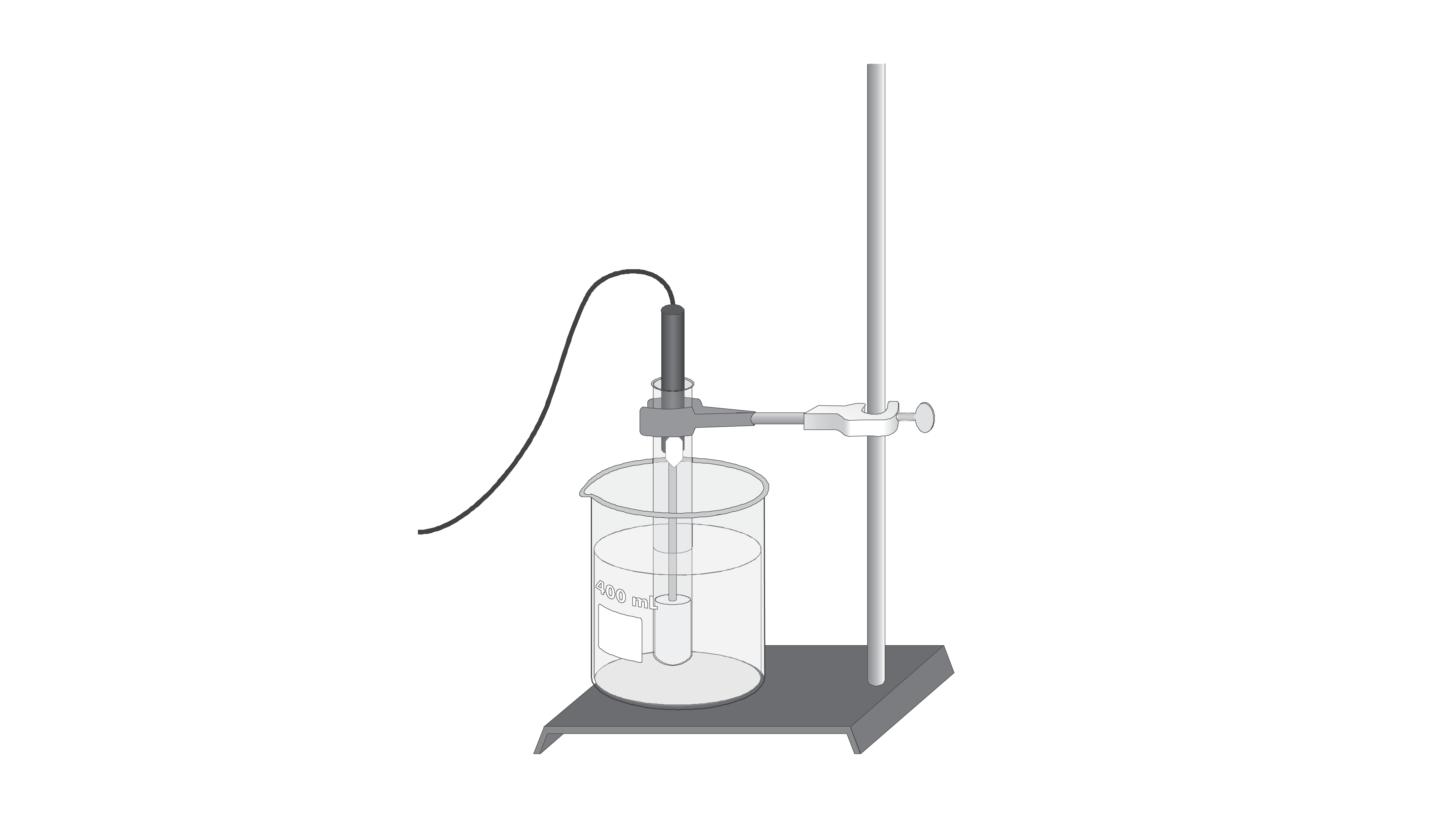
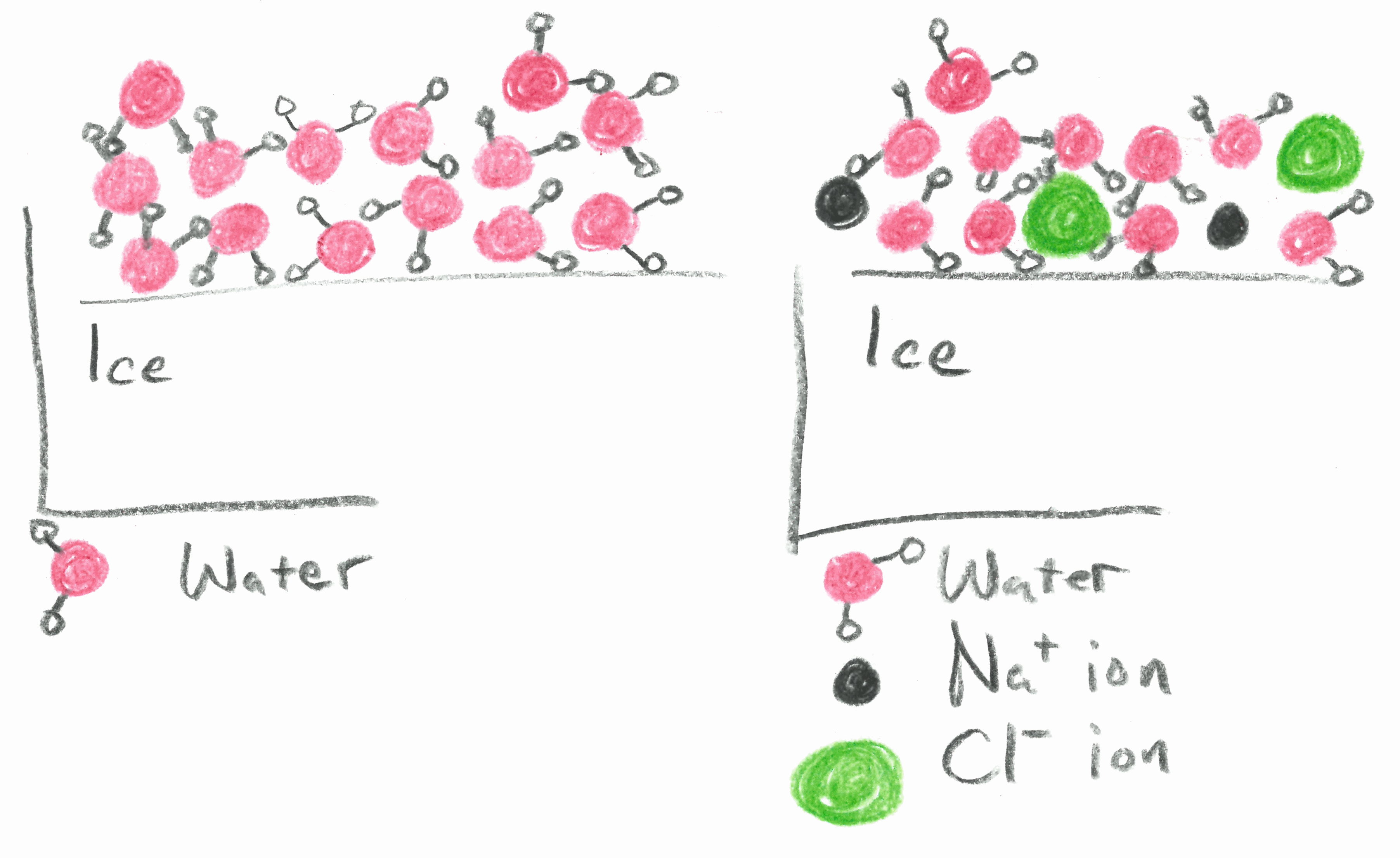
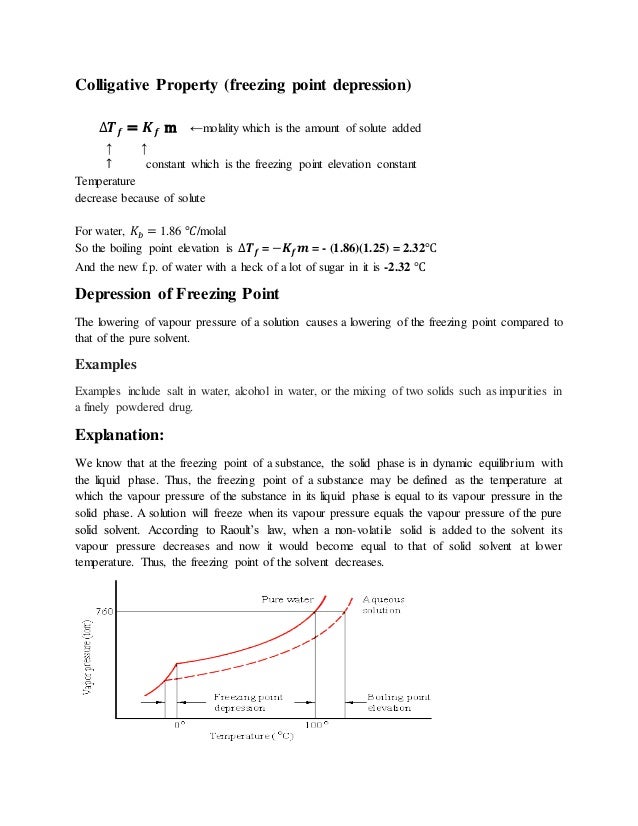
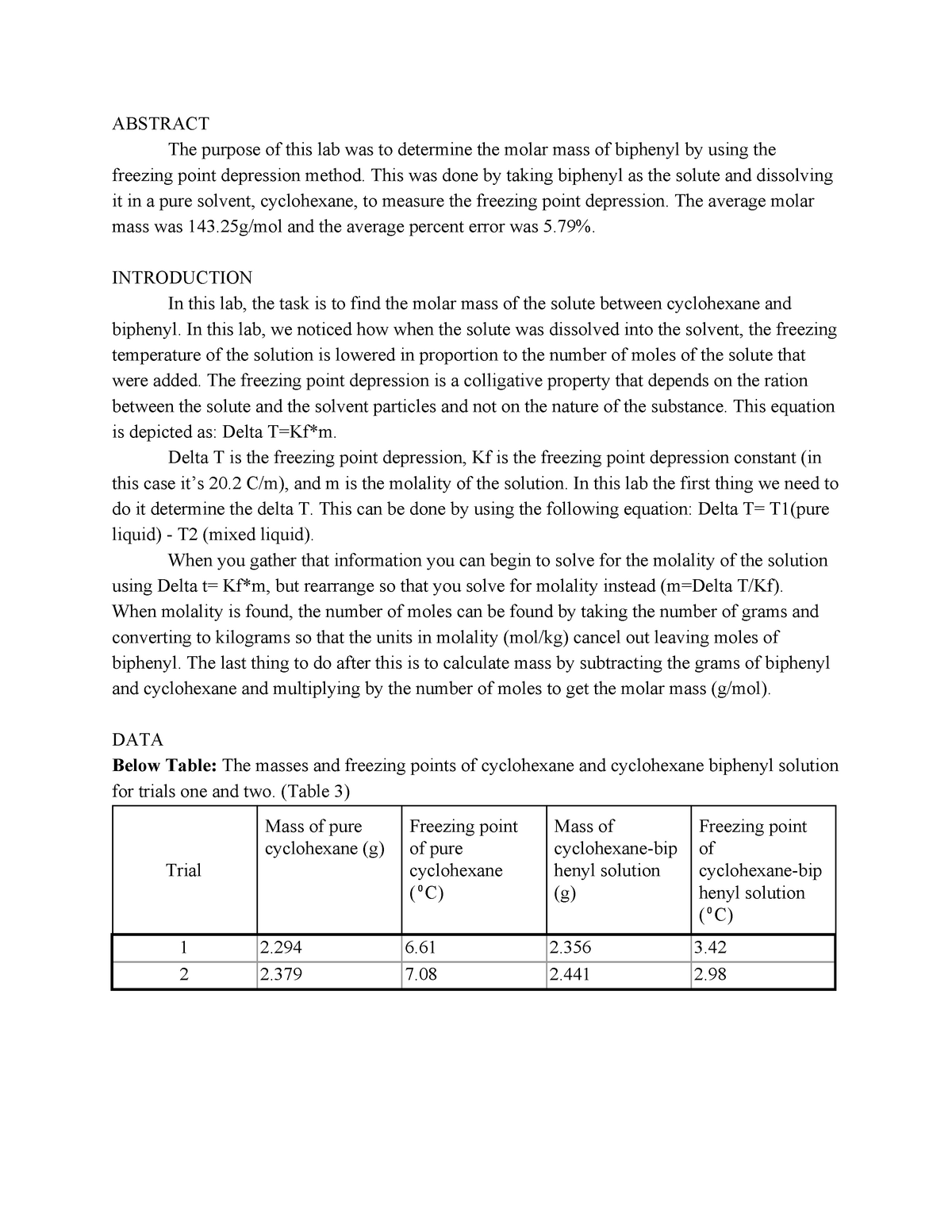
This means that the temperature drop so how much the freezing point lowers does not depend on the type of component added the solute.
Some important uses of freezing point depression are listed below.
Download Freezing point depression Free HD
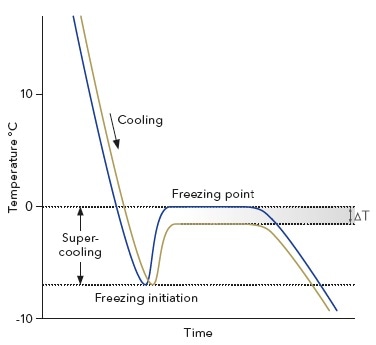
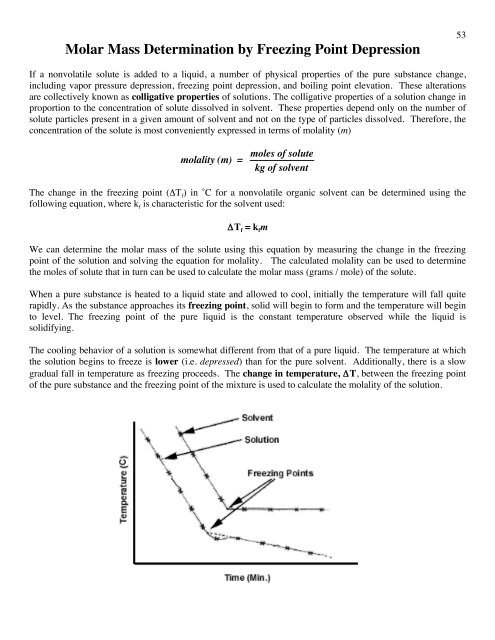
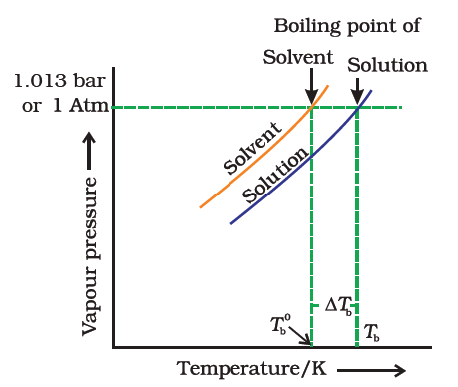
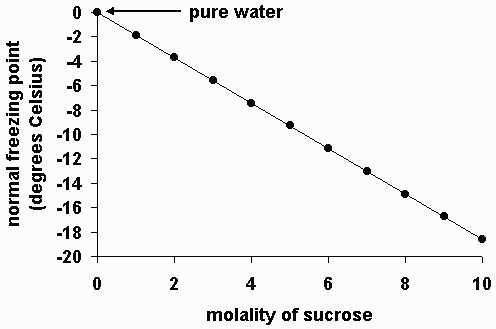
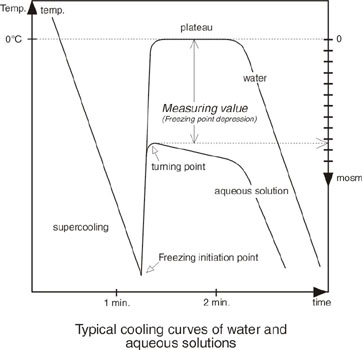
Freezing point depression. On the graph the freezing point depression is represented by. Freezing point depression osmometry is however the most preferred in distinct contexts. Freezing point depression is one of the colligative properties of matter which means it is affected by the number of particles not the chemical identity of the particles or their mass. Colligative properties depend on the number of particles present not on the type of particles or their mass.
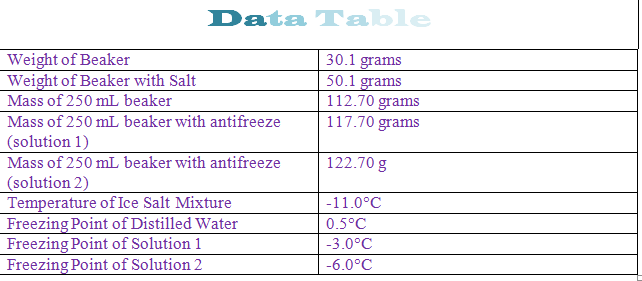
The osmometry is the most preferred in among other areas pharmaceutical quality control laboratories and in clinical chemistry. Freezing point depression is a colligative property observed in solutions that results from the introduction of solute molecules to a solvent. Uses of freezing point depression. Freezing point depression is the decrease of the freezing point of a solvent on the addition of a non volatile soluteexamples include salt in water alcohol in water or the mixing of two solids such as impurities into a finely powdered drug.
So for example if both calcium chloride cacl 2 and sodium chloride nacl completely dissolve in water the calcium chloride would lower the freezing point more than the sodium chloride because it would produce three. Freezing point depression is the phenomena that describes why adding a solute to a solvent results in the lowering of the freezing point of the solvent. When a substance starts to freeze the molecules slow down due to the decreases in temperature and the intermolecular forces start to take over. In the past it has been used to assess the osmotic strength of a colloid and solutions.
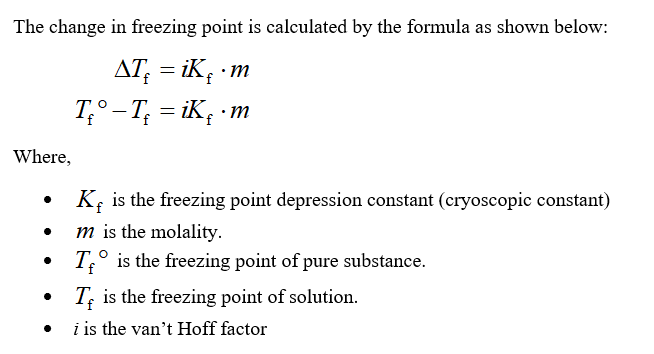
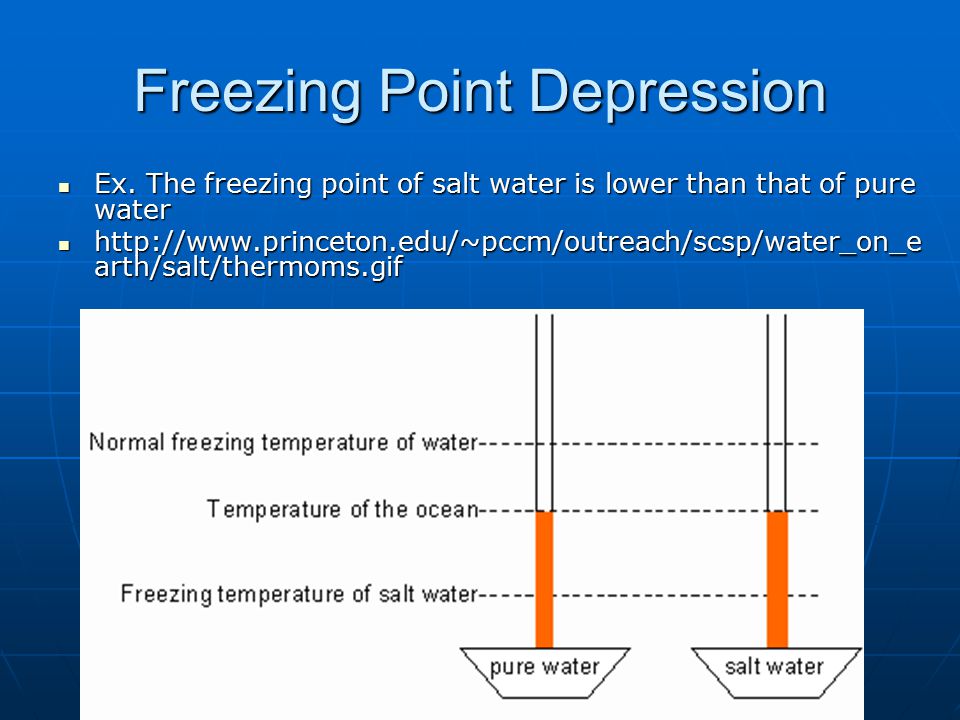
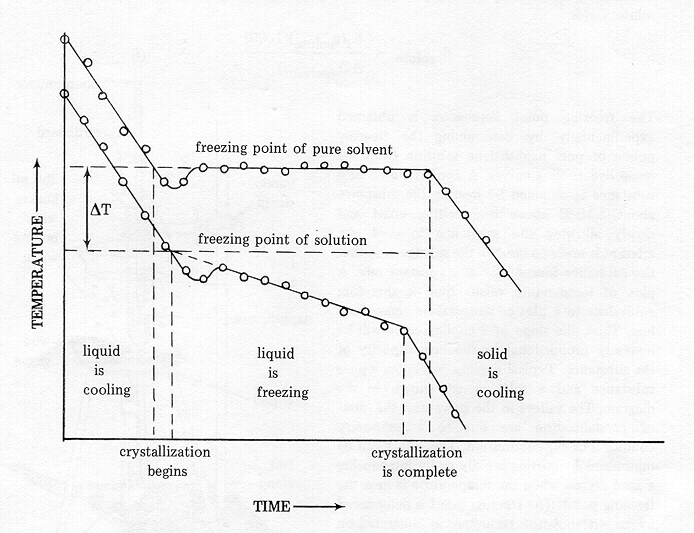
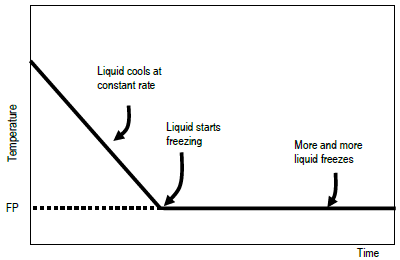
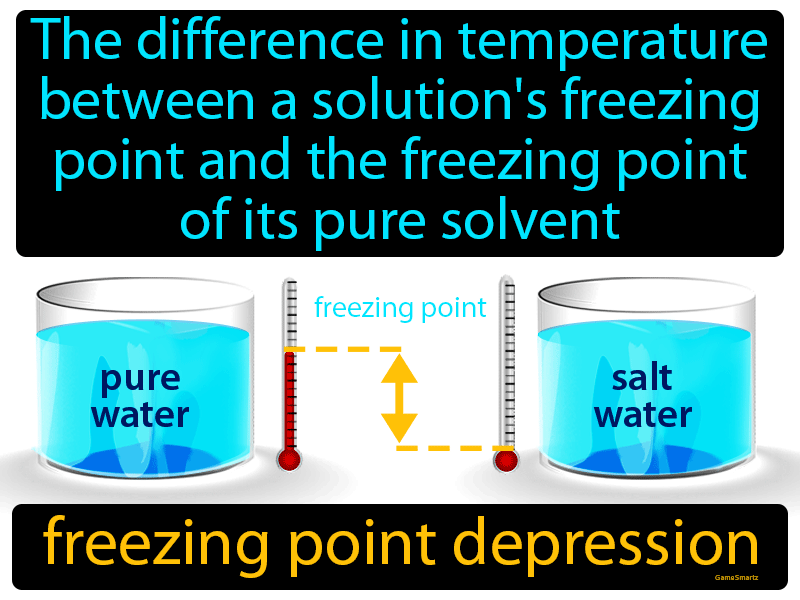
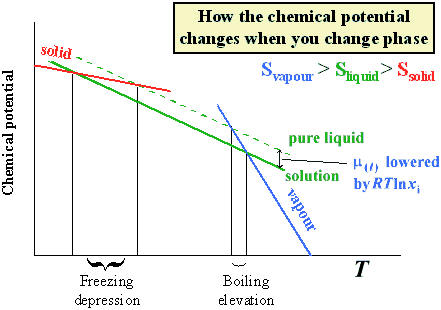
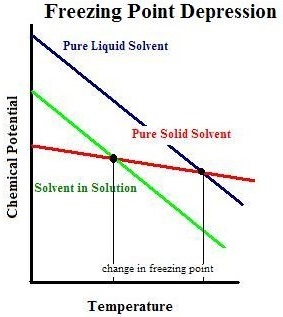
The freezing point depression is a so called colligative property. When a solute is added to a solvent its freezing point is lowered from the original value of the pure solvent. The freezing point depression is the difference in temperature between the freezing point of the pure solvent and that of the solution. This means that a solution must be cooled to a lower temperature than the pure solvent in order for freezing to occur.
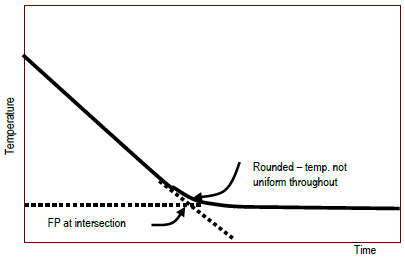
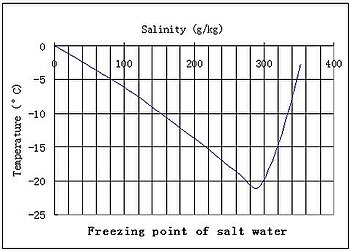
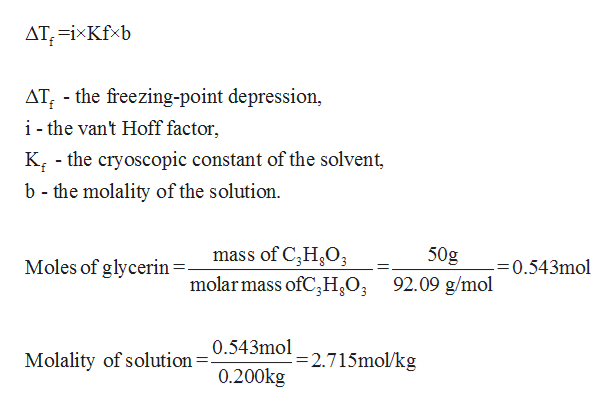
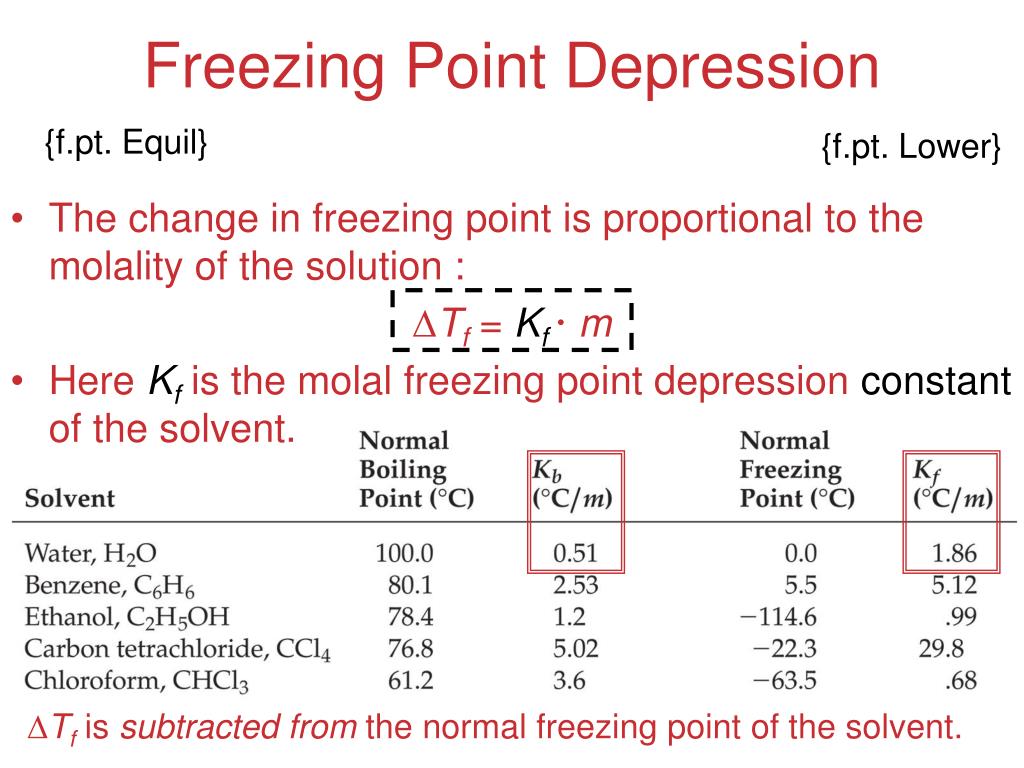
The vapor pressure of a solution blue is lower than the vapor pressure of a pure solvent pink. The freezing points of solutions are all lower than that of the pure solvent and is directly proportional to the molality of the solute. The freezing point of the solvent in a solution changes as the concentration of the solute in the solution changes but it does not depend on the identity of. Deltatf tfsolvent.
Freezing point depression is a colligative property of matter.
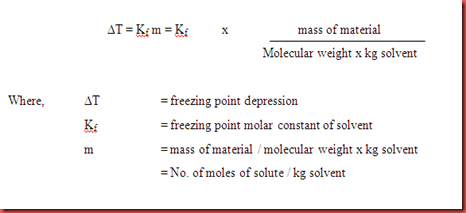


































/close-up-of-ice-cubes-against-white-background-603078349-5829fe673df78c6f6a20deff.jpg)